
Cinnamoyl chloride
Category:
keyword:
Cinnamoyl chloride
Product name:Cinnamoyl chloride
Synonyms:
(2E)-3-Phenyl-2-propenoyl chloride;3-phenyl-2-propenoylchlorid;3-phenyl-acryloylchloride;beta-Phenylacryloyl chloride;Phenylacrylchloride;
Phenylacrylyl chloride;Trans-cinnamoyl chloride
CAS:102-92-1
MF:C9H7ClO
MW:166.60
ASSAY:≥98%
Package:25kg Plastic Drum
Product name:Cinnamoyl chloride
Synonyms:
(2E)-3-Phenyl-2-propenoyl chloride;3-phenyl-2-propenoylchlorid;3-phenyl-acryloylchloride;beta-Phenylacryloyl chloride;Phenylacrylchloride;
Phenylacrylyl chloride;Trans-cinnamoyl chloride
CAS:102-92-1
MF:C9H7ClO
MW:166.60
Appearance: white or yellow crystal
ASSAY:≥98%
Package:25kg Plastic Drum
Water solubility:soluble in petroleum ether, carbon tetrachloride and hot ethanol, insoluble in water. Slow decomposition in water.
Production preparation:It is obtained by the reaction of sodium cinnamate and oxalyl chloride. Prepare a solution of diacetyl chloride and benzene, add the dried sodium cinnamate several times under the stirring from time to time, and add it again after the reaction emits carbon dioxide. Then reflow for 2h. After slightly cooling, filter out the produced sodium chloride and trace unreacted sodium cinnamate. The filtrate is first evaporated to remove benzene under normal pressure, and then distilled under reduced pressure to collect 131 ℃ (1.47kPa) fraction to obtain the product with a yield of 75-90. When purification is required, carbon tetrachloride can be used for recrystallization. Cinnamoyl chloride was prepared by the reaction of cinnamic acid and dichloro sulfoxide.
Storage method:sealed, ventilated, dry, away from fire, etc.
Uses:organic synthesis intermediate, reagent for determination of trace water.
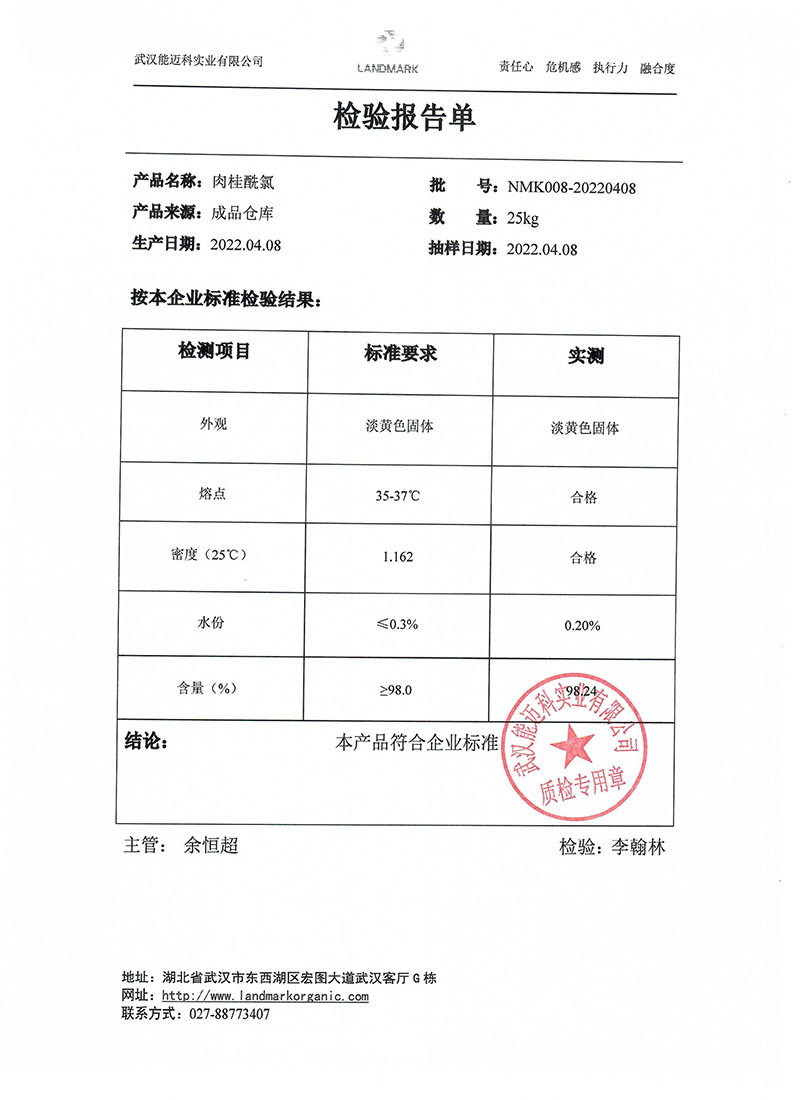
1. For organic synthesis intermediates
2. Reagent for determination of trace moisture
Product name:Cinnamoyl chloride
Synonyms:
(2E)-3-Phenyl-2-propenoyl chloride;3-phenyl-2-propenoylchlorid;3-phenyl-acryloylchloride;beta-Phenylacryloyl chloride;Phenylacrylchloride;
Phenylacrylyl chloride;Trans-cinnamoyl chloride
CAS:102-92-1
MF:C9H7ClO
MW:166.60
Appearance: white or yellow crystal
ASSAY:≥98%
Package:25kg Plastic Drum
Water solubility:soluble in petroleum ether, carbon tetrachloride and hot ethanol, insoluble in water. Slow decomposition in water.
Production preparation:It is obtained by the reaction of sodium cinnamate and oxalyl chloride. Prepare a solution of diacetyl chloride and benzene, add the dried sodium cinnamate several times under the stirring from time to time, and add it again after the reaction emits carbon dioxide. Then reflow for 2h. After slightly cooling, filter out the produced sodium chloride and trace unreacted sodium cinnamate. The filtrate is first evaporated to remove benzene under normal pressure, and then distilled under reduced pressure to collect 131 ℃ (1.47kPa) fraction to obtain the product with a yield of 75-90. When purification is required, carbon tetrachloride can be used for recrystallization. Cinnamoyl chloride was prepared by the reaction of cinnamic acid and dichloro sulfoxide.
Storage method:sealed, ventilated, dry, away from fire, etc.
Uses:organic synthesis intermediate, reagent for determination of trace water.

1. For organic synthesis intermediates
2. Reagent for determination of trace moisture
Product name:Cinnamoyl chloride
Synonyms:
(2E)-3-Phenyl-2-propenoyl chloride;3-phenyl-2-propenoylchlorid;3-phenyl-acryloylchloride;beta-Phenylacryloyl chloride;Phenylacrylchloride;
Phenylacrylyl chloride;Trans-cinnamoyl chloride
CAS:102-92-1
MF:C9H7ClO
MW:166.60
Appearance: white or yellow crystal
ASSAY:≥98%
Package:25kg Plastic Drum
Water solubility:soluble in petroleum ether, carbon tetrachloride and hot ethanol, insoluble in water. Slow decomposition in water.
Production preparation:It is obtained by the reaction of sodium cinnamate and oxalyl chloride. Prepare a solution of diacetyl chloride and benzene, add the dried sodium cinnamate several times under the stirring from time to time, and add it again after the reaction emits carbon dioxide. Then reflow for 2h. After slightly cooling, filter out the produced sodium chloride and trace unreacted sodium cinnamate. The filtrate is first evaporated to remove benzene under normal pressure, and then distilled under reduced pressure to collect 131 ℃ (1.47kPa) fraction to obtain the product with a yield of 75-90. When purification is required, carbon tetrachloride can be used for recrystallization. Cinnamoyl chloride was prepared by the reaction of cinnamic acid and dichloro sulfoxide.
Storage method:sealed, ventilated, dry, away from fire, etc.
Uses:organic synthesis intermediate, reagent for determination of trace water.

1. For organic synthesis intermediates
2. Reagent for determination of trace moisture
Related Products
Welcome to leave an inquiry message. If you need to apply for samples, please fill in the details, and we will contact you in time!


